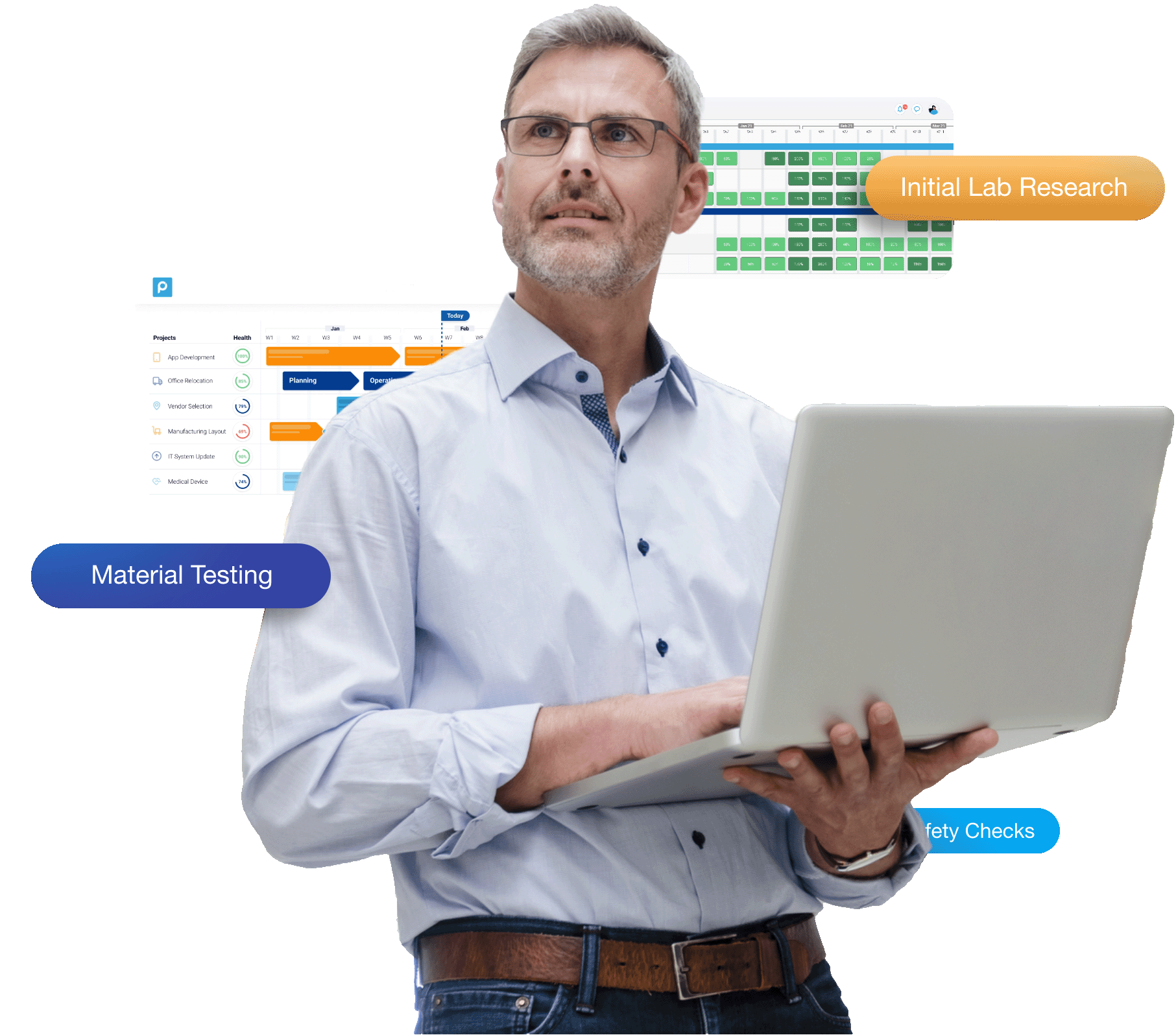
The 4 Biggest Challenges for Biotech & Pharma Project Managers

Project managing an app update or website redesign is one thing. Managing the release of a new medical device or pharmaceutical product is a whole other ball game.
Each brings its own risks and challenges, of course. But biotech and pharma project managers face an entirely unique — and high-stakes — set of obstacles that can impact millions of lives across the globe.
As Dr. Susan Macdonald, principal and founder of Sixteen Hands Biopharma Consulting, said: “Because there are so many unknowns in drug discovery, I see differences in the precision of timelines, predictions of probabilities of success, and resource management in biotech, compared to engineering, IT and manufacturing. In fact, I would say that one of the hardest things to do in biotech project management is put together an integrated timeline, because there are so many variables and unknowns.”
That’s why the 18th annual pharma & biotech Why Summit exists: to dig deep into project management and unpack new tools and strategies for future innovation. Filled with expert keynotes, case studies, and networking opportunities, this event can be a vital resource for anyone navigating these fast-changing fields.
So, ahead of this upcoming Why Summit from April 25-26 in Philadelphia, we’re unpacking the four biggest challenges facing PMOs in biotech and pharma today — along with solutions to help streamline your project management operation.
1. Scheduling compliance and approvals
Biotech projects don’t just have to be approved by stakeholders and executives. They may also have to meet specific regulations set by the U.S. Food & Drug Administration (FDA), and other government bodies. And many of these regulations are still evolving.
In December 2022, for example, the White House Office of Science and Technology Policy (OSTP) announced a request for data to help “identify ambiguities, gaps, inefficiencies, or uncertainties” in the current regulation framework for biotech. Meaning, they’re still looking for the newest info to help update those regulations.
As Dr. Macdonald said, “Changes in regulatory, commercial or internal financial environments and unexpected results from pharmacology, toxicology or clinical studies can all require a rapid change in course […] That is when you really need to bring your problem-solving skills to bear.”
That’s also why it’s so important for biotech PMOs to use an adaptive project management platform — one that lets them easily move milestones without upsetting their whole timeline.
2. Breaking down data silos
It’s no secret: the pharmaceutical industry has a data silo problem. Product development is spread across multiple teams — from design and manufacturing to quality assurance and distribution — each with its own data sets. On top of that, research data is often kept locked inside departments to protect consumer privacy and even prevent other companies from developing competing products.
As a result, pharma companies may be left with tons of duplicated, outdated, or redundant data — which only slows down progress and raises costs. According to a recent survey by Aspen Technology, over half of senior decision-makers at large pharma companies said data silos hindered collaboration in their organization.
There are many potential solutions to this problem — from AI and blockchain technologies to linkable data infrastructures. But at its core, the answer to the silo problem is that all teams must have easy, immediate, and secure access to real-time data. Only then can they keep data updated across the organization and have the information they need to make strategic decisions in a moment’s notice.

The Why Summit is devoting at least two sessions entirely to the topic of resource management. And it’s easy to see why — because this is one of the biggest challenges for today’s biotech and pharma PMOs. Especially when you need people with expertise in specific areas, securing the right talent is essential to success.
With proper resource management, PMOs can help optimize costs, cut down timelines, and prevent burnout. As the description for one of the panels reads: “PMO organizations embracing resource management effectively maintain their competitive edge in times of resource constraints, skills scarcity, flat budgets, and ambitious R&D agendas.”
And this doesn’t just apply to big pharma organizations with thousands of employees. Project and resource management can make or break companies of any size. As Dr. Macdonald put it, “I see project management becoming more accepted and better appreciated earlier in the drug discovery and development process and by more companies […] I think it is being recognized that if you are applying resources to something, that ‘something’ should be well managed.”
4. Seeing the full picture of your portfolio
Managing one biotech or pharma project alone can be a massive undertaking. You’re left juggling a slew of stakeholders, milestones, and meetings, and constantly playing catch-up with last-minute changes. Or, as we like to call it, playing Project Management Whack-a-Mole. Now when you add other projects into the mix — each with their own deliverables — managing that entire portfolio can seem nearly impossible.
“To me, a drug discovery and development program is a bit like a puzzle,” Dr. Macdonald said. “Each task required to achieve the end goal must be put together in the right place, at the right time […] Then, you must step back and look at the picture to make sure there aren’t any holes, and that the picture is clear, correct, and complete.”
That’s why it’s so important to use a project management platform that gives you a bird’s-eye-view of your entire portfolio. So you have the tools to drill down into each daily task, but you also have the freedom to zoom out at any time and see the bigger picture. This is especially crucial for PMOs in biotech and pharma, since they’re dealing with nuanced permissions, regulations, and specializations, while also working to deliver products that could make an impact on a massive scale.
Looking to optimize your project management strategy in the fast-paced world of bio and pharma? Book a demo with Proggio to get started!